Paper mills are infiltrating medical literature at an alarming rate, threatening evidence-based medicine, risking patient safety and undermining pharmaceutical innovation. This article examines how these operations exploit the scholarly ecosystem, their devastating consequences, and how the pharma industry can lead the fight to protect research integrity. By uniting stakeholders through initiatives such as United2Act, we can protect the credibility of science that saves lives.
The growing threat of paper mills
Paper mills – organizations or individuals that aim to profit from the creation, sale, peer review and/or citation of manuscripts at scale – have emerged as a serious threat to the integrity of scientific literature. Their output contaminates the scientific record, jeopardizing the ability of stakeholders to make accurate, evidence-based decisions regarding drug development and patient care. Paper mills pose a direct threat to the pharma industry, for which research integrity is paramount for maintaining public trust.
Paper mills exploit the pressure to publish that many researchers experience in academic settings. They sell authorship, fabricate data, and manipulate peer review and citations. They operate as sophisticated ‘fake science factories’, producing everything from hypotheses to results.
Paper mills may operate by:
- fabricating manuscripts (often with the support of artificial intelligence [AI] tools)
- offering authorship for a fee
- manipulating peer review through fake reviewer identities, or bribing editors and reviewers
- publishing in predatory journals with lax ethical standards.
Figure 1. How paper mills work
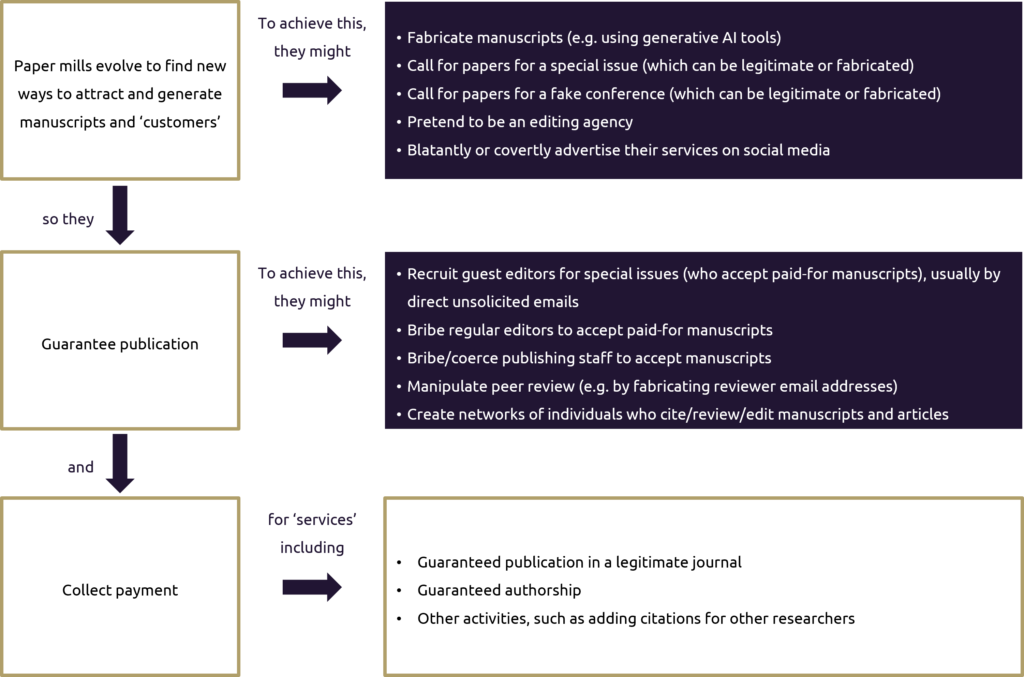
AI, artificial intelligence.
The hallmarks of paper mill outputs are often subtle. Many feature implausible or recycled data, manipulated images or carefully disguised nonsensical text. Paper mills often sell authorship or prewritten manuscripts to researchers who feel under pressure to publish to support their career progression. Others, especially those unfamiliar with these scams, are lured in by promises of quick authorship and citations, but the fallout is severe.
How paper mills undermine pharmaceutical innovation and patient safety
Recent estimates suggest that paper mills produce up to 2% of all papers, and up to 3% of papers in biomedical fields.1 Paper mills have rapidly become one of the leading causes of retractions in academic publishing creating an issue that strikes at the foundation of evidence-based medicine and pharmaceutical innovation.
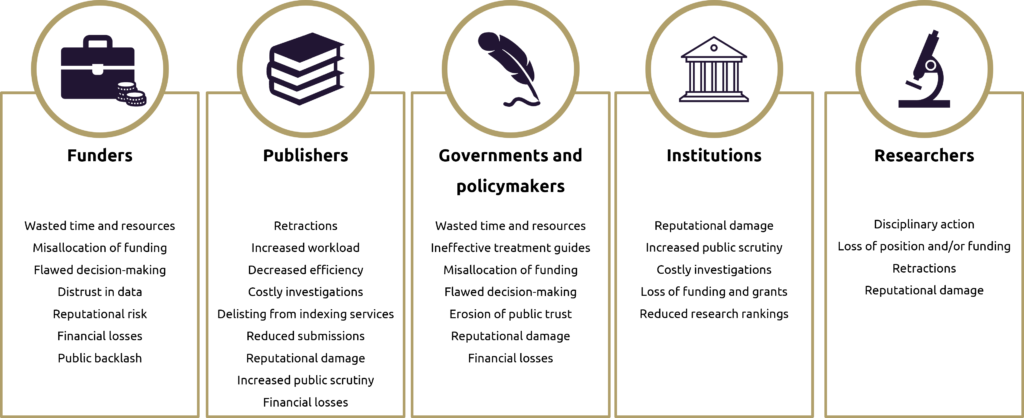
For pharma companies, the consequences extend beyond academic integrity; they can directly affect patient safety and business outcomes. Consider the following scenarios:
- Drug development programmes informed by falsified research could lead to costly late-stage failures.
- Clinical practice guidelines influenced by fraudulent studies might recommend ineffective or unsafe treatments
- Regulatory decisions based on compromised literature could result in approvals that are later withdrawn.
These scenarios undermine the principles of honesty, integrity, rigour, transparency, fairness, respect and accountability that are expected of the pharma industry and could erode public trust in the sector.
Figure 2. The potential consequences of paper mills for stakeholders across the biomedical research lifecycle
United against paper mills: collective action for preserving integrity
It’s clear that no individual stakeholder can solve this problem alone, which is why United2Act has brought together research institutions, publishers, researchers, universities, and industry leaders to combat the threat from paper mills. Our comprehensive approach includes five working groups with complementary objectives to tackle this crisis.
Working group 1: increase education and awareness
Objective: increase awareness and prevention of paper mill fraud by developing and communicating resources. Start with a training toolkit that draws on existing guidance and makes it specific to paper mills.
Working group 2: improve post-publication corrections
Objective: investigate and agree ways to improve communication with people who report misconduct to journals, and agree ways to speed up the correction of the literature when misconduct is discovered.
Working group 3: catalyse research on paper mills
Objective: fill the knowledge gap about paper mills. Encourage research that will further reveal the extent of the paper mill problem and how their practices are maturing, and devise actions to disrupt their impact. The first step is a bibliography of existing literature on this topic.
Working group 4: develop trust markers
Objective: define trust markers as they relate to trust in people, organizations, processes and products, to enable cross-sector communication and strategy development.
Working group 5: facilitate dialogue between stakeholders
Objective: enhance awareness and communication among stakeholders through a common vocabulary and by fostering a shared understanding of the environments that allow paper mills to thrive.
To deepen your understanding of paper mills and to explore practical solutions to the challenges they pose, join our upcoming webinar How to recognise, avoid and prevent paper mills on 14 and 15 May 2025. This webinar is open to all involved in research and publication.
A call to action for pharma leaders
The pharma industry must lead the battle against paper mills by:
- Strengthening internal safeguards
- Reinforce manuscript review processes to include the hallmarks of paper mills (e.g. tortured phrases, duplicated images, suspicious authorship).
- Update publication policies to require full transparency in author contributions, and use of generative AI tools.
- Mandate ethics training for all researchers engaged in publication activities.
- Implement verification procedures for external research cited in key development decisions.
- Raising awareness throughout organizations
- Include paper mill detection in publication training for medical affairs teams.
- Educate clinical development staff on how to identify questionable research.
- Brief regulatory teams on strategies to evaluate literature integrity.
- Maintain updated guidance for addressing suspicious publications.
- Supporting multi-stakeholder initiatives
- Join initiatives such as United2Act.
- Share best practices with industry consortia, such as Open Pharma.
- Allocate appropriate resources to research integrity projects.
- Amplify messages about the risks of paper mills.
The stakes could not be higher
Paper mills erode trust in science – a trust that patients, clinicians, and regulators rely on. This is not hypothetical; it is happening today. You only have to consider the consequences illustrated in our educational resources to understand the problems they catalyse. Fortunately, every one of us in the pharma research community can be a guardian of trust. By uniting as a global community, we can:
- detect fraudulent research faster, before it influences development decisions
- deter paper mills through vigilant scrutiny and reporting
- defend the integrity of the evidence base that drives pharmaceutical advancement.
This is a fight that demands our immediate and sustained attention. This is a fight we must take on.
References
- Van Noorden R. How big is science’s fake-paper problem? Nature 2023;623:466–7. https://doi.org/10.1038/d41586-023-03464-x.
The views expressed in this blog post are those of the author and do not necessarily reflect those of Open Pharma or its Members and Supporters.
Jason Hu is the Chair of Working group 1: increase education and awareness at United2Act a Council Member of COPE, and Director of Research Integrity Engagement at Taylor & Francis.



