Position statement
Pharmaceutical companies, which fund approximately half of all biomedical research,1 are now leaders in the publication and disclosure of research.2,3
However, access to much company-funded research is restricted by journal paywalls.4
We, as Open Pharma, a group of pharmaceutical companies and other research funders, alongside healthcare professionals, regulators, patients, publishers and other stakeholders in healthcare, recognize the importance of publishing research with open access, where papers can be read without payment of a one-off access charge or subscription.
Open access ensures that the highest quality, peer-reviewed evidence is available to anyone who needs it, anywhere in the world. Publishing with open access improves transparency, advances medical science and, we believe, ultimately improves patient care.
Our immediate priority
Our immediate priority is to secure authors publishing company-funded research the same right to publish open access as authors publishing research funded by other sources, so that all research can be made free to read from the date of publication.
This would enable pharmaceutical companies to follow the lead of other research funders in requiring all the research they fund to be published with open access, without impacting on journal choice.5-7
In order to provide publishers the time to adapt their policies and protect their copyright interests, any variant of Creative Commons or equivalent licence could be used.
Our long-term goal
Our long-term goal is to secure authors publishing company-funded research the same terms as authors publishing research funded by other sources, so that all research can be made free to read – and reuse – from the date of publication.
This would enable pharmaceutical companies to follow the lead of other research funders in maximizing the impact of the research they fund on patient health.
Open Pharma is committed to working closely with publishers and pharmaceutical companies to ensure that the gold standard Creative Commons Attribution (CC BY) licence can sustainably be used.8
Acknowledgements
This Open Pharma position statement on open access represents the opinions of the individual members: Catherine Skobe (Pfizer), Chris Rains (Takeda), Chris Winchester (Oxford PharmaGenesis), Julie Newman (Gilead), Lise Baltzer (Novo Nordisk), Sarah Sabir (Oxford PharmaGenesis) and Valerie Philippon (Takeda), and not necessarily those of their individual companies. We thank the members, supporters and followers of Open Pharma for their input and valuable discussion.
Sign up here to endorse the Open Pharma position statement
Endorsements
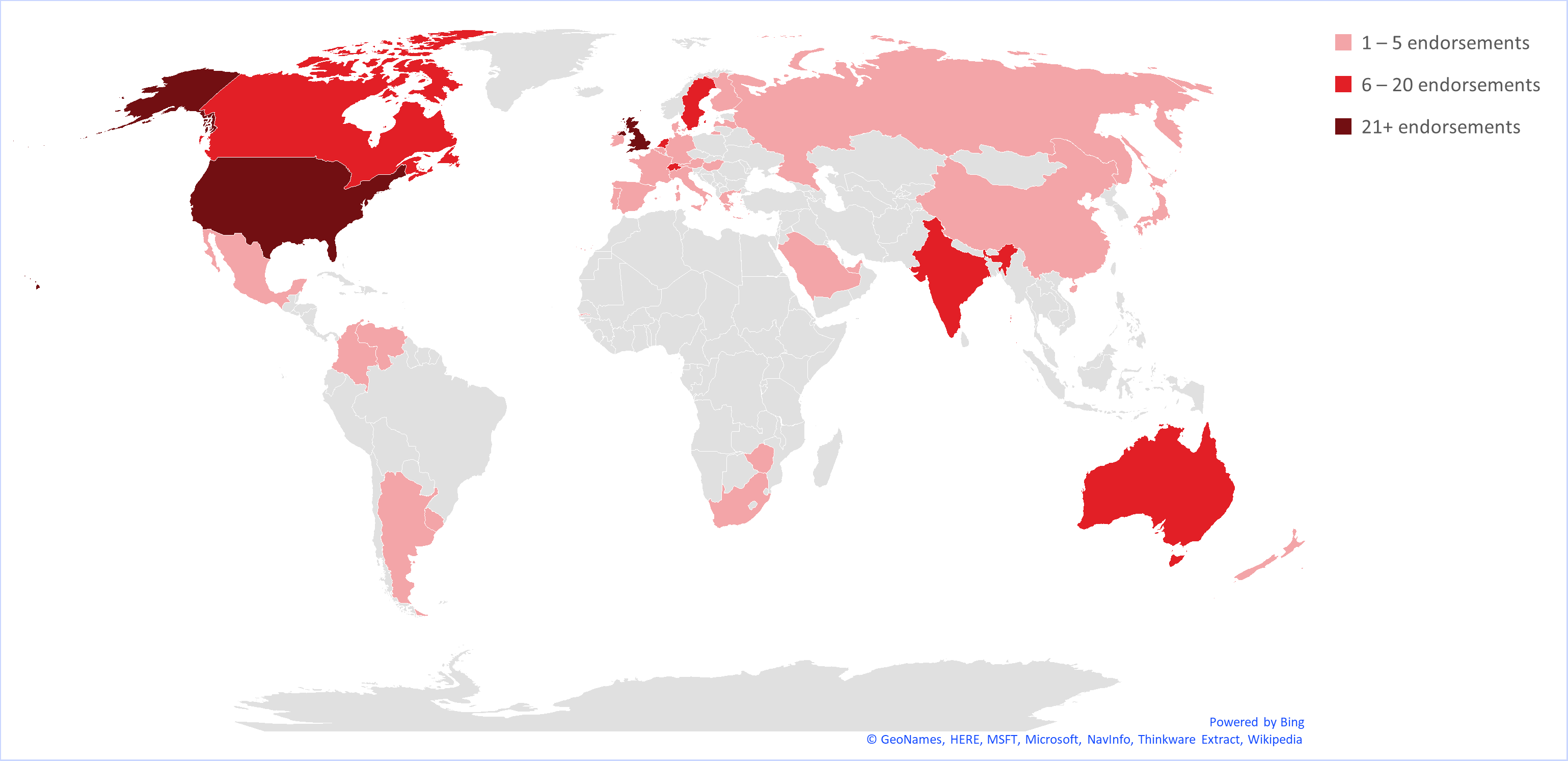
Publishers
Betasciencepress Publishing; ecancer; F1000 Research Ltd.; Frontiers Media SA; Future Science Group; Hindawi; MDPI; Orapuh, Inc.; PLOS; Wiley
Organizations
AIX Consultancy; Ataxia & Me; Autoinflammatory UK; Cambridge Rare Disease Network; Canadian Organization for Rare Disorders; Centro Español de Investigación Farmacoepidemiológica (CEIFE); Cinclus Pharma Holding AB; DSL Consulting, LLC; Epi-Fit; FibroFlutters Patient Advocacy; Galapágos NV; Inspiring STEM; International Kidney Cancer Coalition; Ipsen; KAN Consulting MON. I.K.E; Kidney Research UK; M-CM Network; M-Spective Ltd; Observational and Pragmatic Research Institute Pte; Outcomes Positive, Inc; Oxford Health Policy Forum; Oxford PharmaGenesis; Pfizer; Pedalling4ACure; SCN2A Australia; Scott Pharma Solutions; Scriva Medical Communications; Sequoia Medical Communications Ltd; Solanum Medical Communications Ltd; SUDEP Action; The Aarskog Foundation; ThinkSCIENCE, Inc.; Zimbabwe Evidence Informed Policy Network
Individuals
Michelle Koutsantonis, AbbVie; Andrew Xia, AIX Consultancy; Kenneth Pomerantz, Alexion Pharmaceuticals; Larisa Miller, Alexion Pharmaceuticals; Mary Kunjappu, Alexion Pharmaceuticals; Mario Palencia, Aparito; Börje Wernersson, formerly of AstraZeneca; Saga Johansson, formerly of AstraZeneca; Alan Thomas, Ataxia & Me; Scott Armit, Ataxia & Me; John Wallace, Autoinflammatory UK; Gavin Giovannoni, Barts and the London School of Medicine and Dentistry; Roland J.W. Meesters, Betasciencepress Publishing; Andrew Balas, Biomedical Research Innovation Laboratory, Augusta University; Durhane Wong-Rieger, Canadian Organization for Rare Disorders; Luis Alberto García Rodríguez, Centro Español de Investigación Farmacoepidemiológica (CEIFE); Peter Unge, Cinclus Pharma Holding AB; Cipriano Martinez, Cipriano Martinez LTD; Kathleen Boyle, Cleveland Clinic; Val Tate, Communications Consultant; Glenda Hardy, Continuing Medical Education; Raj Kadadai, Cure SCA3; Inmaculada Silos-Santiago, Decibel Therapeutics; Douglas Levine, DSL Consulting; Brian S. Alper, EBSCO Information Services; Katie Foxall, ecancer; Laure Lacoin, Epi-Fit; Adriana Rocha, Freelance; Andrea Rossi, Freelance; Paolo Rega, Freelance; Coral Milburn, Green Templeton College; Denise Lievesley, Green Templeton College; Wilbur Liu, Haalthy; Stephanie Weber, Idorsia Pharmaceuticals Ltd; Laverne Mooney, Independent Consultant; Ivan Gonzalez, Independent Medical Consultant; Jane Potee, Ingenta; Linda Edmonson, Innovative Strategic Communications; Tim Day, Innovative Strategic Communications; Martin Delahunty, Inspiring STEM; Anna Baikova, Ipsen; Chandan Sabat, Jaypee Brothers Medical Publisher; Kamila Novak, KAN Consulting MON. I.K.E; Nicole Bezuidenhout, Karolinska Institute; Kyrstie Sutcliffe, K J Consulting Ltd; Károly Róbert Kulich, Kulich Consulting; Baiba Ziemele, Latvian Alliance of Rare Diseases; Lauren Xie, Lively Worlds; Jan Schoones, LUMC; Egon Willighagen, Maastricht University; Julia Mawer, Max Planck Institute; Christy Collins, M-CM Network; Gina D’Angelo, Magraeme Consulting LLC; Grigorios Leontiadis, McMaster University; Franck Vazquez, MDPI; Rita Moreira de Silva, Medscape; Andrew Worsfold, M-Spective Ltd; Alben Sigamani, Narayana Health; Michael De Rosa, National Ataxia Foundation; Peter Llewellyn, NetworkPharma Ltd.; Ana-Madalina Ion, Newcastle University; Pali Hungin, Newcastle University; Stephen Canham, Novartis; Klaus Boberg, Novo Nordisk; Heather Piwowar, Our Research; Tracy Bunting, Outcomes Positive, Inc; Serena Vanzan, Oxford Brookes University; Emma Georgiou, Oxford Health Policy Forum; Kajsa Wilhelmsson, Oxford Health System Reform Group; Adam Hargreaves, Oxford PharmaGenesis; Adele Buss, Oxford PharmaGenesis; Adeline Rosenberg, Oxford PharmaGenesis; Agnieszka Ragan, Oxford PharmaGenesis; Aimee Jones, Oxford PharmaGenesis; Alison Hillman, Oxford PharmaGenesis; Anja Becher, Oxford PharmaGenesis; Akhil Bansal, Oxford PharmaGenesis; Bobby Thompson, Oxford PharmaGenesis; Carly Sellick, Oxford PharmaGenesis; Cassidy Fiford, Oxford PharmaGenesis; Caroline Leitschuh, Oxford PharmaGenesis; Chloe Fletcher, Oxford PharmaGenesis; Christian Eichinger, Oxford PharmaGenesis; David Lugmayer, Oxford PharmaGenesis; Elin Bevan, Oxford PharmaGenesis; Emma Bolton, Oxford PharmaGenesis; Emma Butcher, Oxford PharmaGenesis; Francesca Ounsworth, Oxford PharmaGenesis; Gabriella Ribenfors, Oxford PharmaGenesis; George Gomez, Oxford PharmaGenesis; Helen Simpson, Oxford PharmaGenesis; Helen Stimpson, Oxford PharmaGenesis; Jessica Hardy, Oxford PharmaGenesis; Joana Osório, Oxford PharmaGenesis; Joanna Donnelly, Oxford PharmaGenesis; Joshua Dow, Oxford PharmaGenesis; Joseph Hawkins, Oxford PharmaGenesis; Julie Beeso, Oxford PharmaGenesis; Karol Bociek, Oxford PharmaGenesis; Lisa Deakin, Oxford PharmaGenesis; Maria Farrell, Oxford PharmaGenesis; Mark Elms, Oxford PharmaGenesis; Mark Rolfe, Oxford PharmaGenesis; Michelle Antoni, Oxford PharmaGenesis; Michael Molloy-Bland, Oxford PharmaGenesis; Nicolas Bertheleme, Oxford PharmaGenesis; Paul Farrow, Oxford PharmaGenesis; Rebecca Prince, Oxford PharmaGenesis; Richard White, Oxford PharmaGenesis; Robert Walsh, Oxford PharmaGenesis; Sally Bardell, Oxford PharmaGenesis; Sarah Sabir, Oxford PharmaGenesis; Shawna Matthews, Oxford PharmaGenesis; Sophie Nobes, Oxford PharmaGenesis; Steph Macdonald, Oxford PharmaGenesis; Steven Inglis, Oxford PharmaGenesis; Tamsyn Stanborough, Oxford PharmaGenesis; Tamzin Gristwood, Oxford PharmaGenesis; Tim Ellison, Oxford PharmaGenesis; Tim van Hartevelt, Oxford PharmaGenesis; Tim Koder, Oxford PharmaGenesis; Tomas Rees, Oxford PharmaGenesis; Trevor Sills, Oxford PharmaGenesis; Victoria Ankrah, Oxford PharmaGenesis; Zoe Watts, Oxford PharmaGenesis; Graham Shelton, Oxford University Hospitals Foundation; Magdalen Wind-Mozley, Oxford Vaccine Group; Charles Boyce, patient; Emily Medina, patient; Jeannie Smithball, patient; Danielle Grover, patient advocate; Elizabeth Kinder, patient advocate; Richard Stephens, patient advocate; Tracy Zervakis, patient advocate; Trishna Bharadia, patient advocate; Rosario Strano, Pedalling4Cure; Adam Watson, Pfizer; Angela Sykes, Pfizer; Arjun Krishnakumar, Pfizer; Barbara Pritchard, Pfizer; Berkeley Phillips, Pfizer; Beth Whann, Pfizer; Christine Chun, Pfizer; Courtney Leo, Pfizer; Devy Lee, Pfizer; Durell Dsouza, Pfizer; Helen Park, Pfizer; J.R. Meloro, Pfizer; Janet Galliera, Pfizer; Jennifer Ghith, Pfizer; Kripa Madnani, Pfizer; Ksenia Astanina, Pfizer; Luciano Passador, Pfizer; Marcia Wright, Pfizer; Marianne Gandee, Pfizer; Melissa Furtado, Pfizer; Mirtha Marcela Garcia, Pfizer; Nervana Habashy, Pfizer; Nicolas Garnier, Pfizer; Reema Dhoke, Pfizer; Ronika Alexander-Parrish, Pfizer; Sally Dews, Pfizer; Sonia Philipose, Pfizer; Stephanie Dooley, Pfizer; Sweta Samantaray, Pfizer; Tara Moroz, Pfizer; Tatjana Zanki, Pfizer; Terri Craig, Pfizer; Varkha Agrawal, Pfizer; Ellie Challis, PTEN UK & Ireland; Dermot Ryan, Respiratory Effectiveness Group; Rikke Egelund Olsen, Roche; Grannum Sant, Sant Consulting LLC; Christos Petrou, Scholarly Intelligence; Kris Pierce, SCN2A Australia; Susan Scott, Scott Pharma Solutions; Evguenia Alechine, Science Communicator; Jim Gavin, Scriva Medical Communications; David Peters, Sequoia Medical Communications Ltd; Brian Shearer, Shearer Publications LLC; Sophie Berry, Smith & Nephew; John Gonzalez, Solanum Medical Communications Ltd.; Amy Whereat, Speak the Speech Consulting; Laura Paglione, Spherical Cow Group; Jane Hanna, SUDEP; Noor Al-Abood, Taif University; William Spalding, Takeda; Raquel Billiones, Takeda Vaccines; Victoria Woods, Patient and Public Engagement, Thames Valley Cancer Alliance at NHS England; Zbys Fedorowicz, The Care Combine and North West Anglia NHS Foundation Trust; Stuart Taylor, The Royal Society; Caryn Jones, THINK Science; John Pernick, THINK Science; Luiza Erthal, Trinity College Dublin; Steven Merkel, Twist Medical, LLC; Erik Michels, UCB Pharma; Linda Feighery, UCB Pharma; Rachel Giles, Regenerative Medicine Center, UMC Utrecht; Meredith Hays, Department of Medicine, Uniformed Services University; Emmanuel Picavet, Université Paris 1 Panthéon-Sorbonne; Amy Booth, University of Cape Town; University of Oxford; Reggie Raju, University of Cape Town; Martin Rossor, University College London; Stephen Bradley, University of Leeds; Robert Sim, Department of Respiratory Science, University of Leicester; Jukka Ronkainen, University of Oulu; Barbara Xella, University of Oxford; Chas Bountra, Nuffield Department of Clinical Medicine, University of Oxford; Edith Sim, Department of Pharmacology, University of Oxford; Iain Chalmers, Centre for Evidence-Based Medicine, University of Oxford; Makafui Tunde Adebayo, University of Oxford; Shivanki Sahay, University of Oxford; Stevan Harnad, University of Southampton; Álvaro Díaz, Departamento de Biociencias, Universidad de la República, Uruguay; Steve Maingot, Waltham Forest Community and Family Health Services; Claire Gillis, WPP; Ronald Munatsi, Zimbabwe Evidence Informed Policy Network.
References
- Dorsey ER, de Roulet J, Thompson JP et al. Funding of US biomedical research, 2003–2008. JAMA 2010;303:137–43.
- Baronikova S, Purvis J, Southam E et al. Commitments by the biopharmaceutical industry to clinical trial transparency: the evolving environment. BMJ Evidence-Based Medicine 2019:bmjebm–2018–111145.
- Warren M. Big pharma is embracing open-access publishing like never before. Nature, 2019. Available from: www.nature.com/articles/d41586-019-00610-2 (Accessed 3 October 2019).
- Ellison TS, Koder T, Schmidt L, Williams A, Winchester CC. Open access policies of leading medical journals: a cross-sectional study. BMJ Open 2019;9:e028655.
- Collins E. Publishing priorities of biomedical research funders. BMJ Open 2013;3:e004171.
- Baronikova S, Desai SY, Philippon V, Rains CP. An assessment of open access publishing at Shire before and after implementation of the open access publication policy. Curr Med Res Opin 2019;35(Suppl 2):31.
- Lang H, Winchester CC, Gattrell W. Open access publishing of research affiliated to Ipsen, 2013–2017: a baseline assessment. Curr Med Res Opin 2019;35(Suppl 2):39.
- Tennant JP, Waldner F, Jacques DC et al. The academic, economic and societal impacts of Open Access: an evidence-based review. F1000Res 2016;5:632.
