Open Pharma has developed a new, free-to-view, online tool that reports open access (OA) publishing rates, access types and OA licences for peer-reviewed medical publications with authors affiliated to pharma companies and universities.
The Open Pharma OA position statement emphasizes the importance of publishing research OA to ensure that high-quality, peer-reviewed evidence is available to anyone who needs it, anywhere in the world and without charge.
The recognized benefits of OA publishing include improved equity in access to medical knowledge and scientific advances, increased research transparency and the potential to foster greater public trust in scientific research (Figure 1). Emerging data from global publishers Taylor & Francis also suggest that research published OA typically has higher reach and impact than comparable paywalled articles of a similar age.1
Figure 1. The benefits of OA publishing
OA, open access.
Motivated by the potential benefits of OA publishing, several major public research funders have implemented mandates in recent years that require grant recipients to publish their findings promptly and OA (UK Research and Innovation, 2021; US government, 2022). In the private sector, similar appreciation of the value of OA publishing has been reflected in OA mandates from the pharmaceutical companies Takeda (formerly Shire; in 2018), Ipsen (in 2019) and Galápagos (in 2020).
It is within this context that Open Pharma has launched the Open Pharma OA dashboard. The free-to-use tool will benchmark OA rates for research with authors affiliated to pharma companies and universities and provide insights into the extent to which growing recognition of OA publishing benefits is translating into changes in practice.
Using source data from the Lens platform, which contains > 200 million scholarly publications, the Open Pharma dashboard reports OA publishing rates for articles with authors affiliated to pharma companies (focusing on the top 40 companies by R&D expenditure) and universities (focusing on the top 40 by Leiden Ranking of scientific performance). Rates can also be viewed stratified by access type and OA licence.
The aim of the dashboard is to provide insights into the current OA landscape and emerging trends and to use these data to inform OA objective setting to facilitate increased uptake and equity in OA publishing across funding sectors.
Further information about the data, including initial benchmarking data (Figure 1), was presented at the 19th Annual Meeting of International Society for Medical Publication Professionals this week in Washington. Our poster was titled Benchmarking open access in publications with authors affiliated to pharma companies and universities (09:00, 24 April 2023; poster #47).2
Click here to view the benchmarking tool.
Figure 2. OA licence rate for pharma and university articlesb
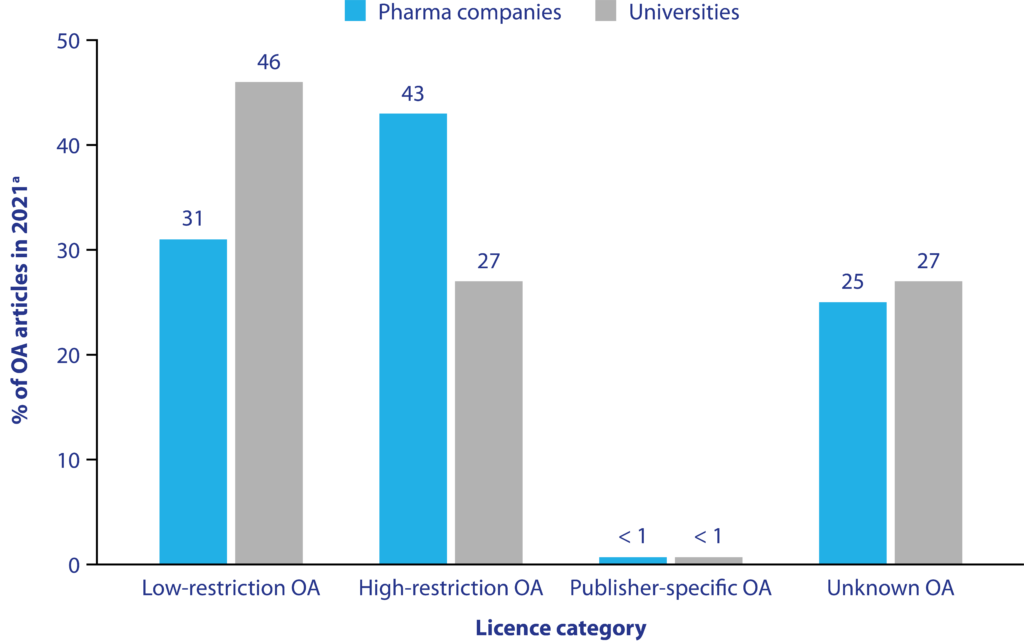
Figure first presented at the 2023 Annual Meeting of ISMPP (poster #47).
aArticles may feature in more than one category of OA licence. Percentages were calculated using the total number of OA articles published in 2021, so the sum of percentages across categories may not be 100%. Low-restriction OA included articles published under CC0, CC BY and public domain. High-restriction OA included articles indexed as CC BY-NC, CC BY-ND and CC BY-SA. Publisher-specific OA included any article with a licence under the authority of a specific publisher. For information on licences, visit the Creative Commons website. bArticles with authors affiliated to either the top 40 pharma companies or the top 40 universities.
ISMPP, International Society for Medical Publication Professionals; OA, open access.
References
- Nagda P et al. The changing open research landscape: a publisher’s perspective. 2023. Available from: https://www.openpharma.blog/blog/accessibility/open-access/the-changing-open-research-landscape-a-publishers-perspective/ (Accessed 25 April 2023).
- Philippon V et al. Benchmarking open access in publications with authors affiliated to pharma companies and universities. Poster 47. Presented at ISMPP US 2023, 24 April 2023. Available from: https://www.congressposter.com/p/47hnk1uoz7ltsm5u (Accessed 25 April 2023).






